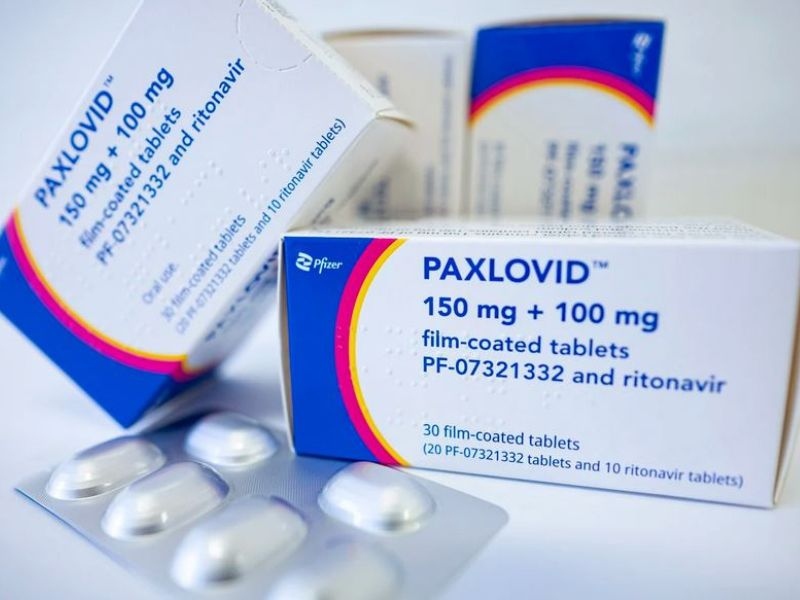
The U.S. Food and Drug Administration gave Pfizer's Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) an emergency use authorization (EUA) for the treatment of mild-to-moderate coronavirus disease (COVID-19)
in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 testing and who Paxlovid is only available with a doctor's prescription. It should be started as soon as possible after a COVID-19 diagnosis and within five days of the first sign of illness.
"Today's approval brings the world's first pill-based treatment for COVID-19, which is a big step forward in the fight against this global pandemic," said Patrizia Cavazzoni, M.D., director of the FDA's Center for Drug Evaluation and Research. "This authorization gives us a new way to fight COVID-19 at a crucial time in the pandemic, when new variants are showing up. It also promises to make antiviral treatment easier to get for people who are at high risk of getting severe COVID-19."
Paxlovid is not allowed to be used to prevent COVID-19 before or after exposure, or to start treatment in people with severe or critical COVID-19 who need to stay in the hospital. Paxlovid is not a replacement for vaccination for people who should get a COVID-19 vaccination and a booster dose. The FDA has approved one vaccine and given permission for others to be made to prevent COVID-19 and the serious health problems that come with it, like hospitalization and death.
The FDA wants everyone to get vaccinated and, if they can, get a booster shot. Find out more about COVID-19 vaccines that have been approved or licensed by the FDA.
How does Paxlovid work!?
Paxlovid is made up of two parts: nirmatrelvir, which blocks a SARS-CoV-2 protein to stop the virus from spreading, and ritonavir, which slows the breakdown of nirmatrelvir so that it stays in the body longer and in higher concentrations. Paxlovid is given as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) that are taken by mouth twice a day for five days. There are a total of 30 tablets in the treatment. It is not okay to take Paxlovid for more than five days in a row.
Difference between EUA and FDA
Giving out an EUA is different from giving out an FDA approval. In order to decide whether or not to issue an EUA, the FDA looks at all of the available scientific evidence and carefully weighs the product's known or possible risks against its known or possible benefits. Based on the FDA's review of all the available scientific evidence, the agency has decided that it is reasonable to think that Paxlovid may help treat mild-to-moderate COVID-19 in patients who have been given permission to use it. The agency has also decided that the known and possible benefits of Paxlovid, when used according to the terms and conditions of the authorization, are greater than the known and possible risks of the product. There are no good, approved alternatives to Paxlovid that can be used to treat COVID-19.
EPIC-HR is a randomized, double-blind, placebo-controlled clinical trial that looked at Paxlovid for treating SARS-CoV-2 infection in adults who were not in the hospital and who had symptoms.
Who Should be treated by Paxlovid
Patients were adults who were 18 years old or older and had a known risk factor for a disease getting worse, or they were 60 years old or older and had a known chronic medical condition. All of the patients had never been vaccinated against COVID-19 and had never had COVID-19 before. The main thing the trial looked at was how many people had to go to the hospital because of COVID-19 or died from any cause during the 28 days of follow-up. When compared to placebo, Paxlovid cut by 88% the number of COVID-19-related hospitalizations or deaths from any cause in people who were treated within five days of their first symptoms and who did not get COVID-19 therapeutic monoclonal antibody treatment. In this study, 1,039 patients got Paxlovid and 1,046 got a fake drug called a placebo. Of these patients, 0.8% of those who got Paxlovid were hospitalized or died during the 28-day follow-up period, while only 6% of those who got the placebo did. The safety and effectiveness of Paxlovid as a COVID-19 treatment are still being looked into.
Some of the possible side effects of Paxlovid are bad taste, diarrhea, high blood pressure, and muscle aches. Using Paxlovid with certain other drugs could lead to drug interactions that could be very bad. Using Paxlovid on people whose HIV-1 infection isn't under control or hasn't been diagnosed could cause HIV-1 to become resistant to drugs. Ritonavir might hurt the liver, so Paxlovid shouldn't be given to people who already have liver disease, liver enzyme problems, or inflammation of the liver.
Because Paxlovid works in part by stopping a group of enzymes from breaking down some drugs, it shouldn't be taken with drugs that depend on those enzymes for metabolism and for which high levels of certain drugs can cause serious or even life-threatening side effects. Paxlovid shouldn't be taken with drugs that, on the other hand, strongly stimulate the same enzymes, which speeds up the breakdown of nirmatrelvir or ritonavir.
This is because lower levels of nirmatrelvir or ritonavir may cause the virus to stop responding to the drug and become resistant to it. Because the effects of these medications last after you stop taking them, you can't start Paxlovid right away after stopping them. See the fact sheet for health care providers for a full list of medicines that shouldn't be taken with Paxlovid.
Who Shouldn't be treated by Paxlovid
Patients who have severe problems with their kidneys or liver should not take Paxlovid. Patients with moderate kidney damage need a lower dose of Paxlovid. Patients who have problems with their kidneys or liver should talk to their doctor about whether or not Paxlovid is right for them.
Under the EUA, healthcare providers, patients, and caregivers must have access to fact sheets that give important information about how Paxlovid can be used to treat COVID-19 as authorized. These fact sheets have information about how to take Paxlovid, possible side effects, drug interactions, and who can prescribe it.








 And then Add to Home Screen.
And then Add to Home Screen.